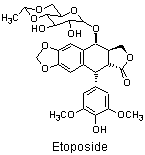
Many antitumour antibiotics have been isolated from natural sources and identified from screens for cytotoxic compounds, such as the NCI 60 cell-line screen.
Many of these compounds interact directly with DNA but do not form covalent bonds. The main interactions are formed in two ways.
The binding of the drug to DNA changes the structure of the double helix. As a consequence, many of the processes required within the cell are inhibited, including DNA directed RNA synthesis and DNA processing by topoisomerase II.
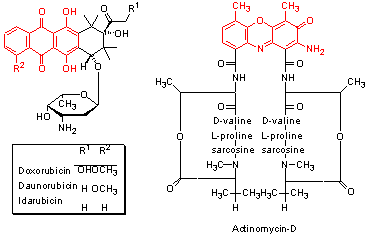
Each cell contains approximately 2m of DNA packed into its nucleus.
The DNA is wound around basic proteins called histones and is tighly coiled or supercoiled.
Sequences of DNA must be unwound or relaxed before they can be read by DNA processing enzymes.
Topoisomerase II or TopoII is one enzyme which does this job.
TopoII temporarily breaks the DNA strand and allows both ends to freely rotate within the enzyme.
This allows the DNA to unwind.
The enzyme then reconnects the two ends leaving a section of relaxed DNA ready for processing.
TopoII inhibitors, e.g. Etoposide,
stabilise the enzyme with the DNA strand cut (the cleavable complex)
long enough for the protein DNA complex to break down leaving a double strand break in the DNA.
The strand breaks formed in this way are capped by the remnants of the TopoII protein and so are more difficult to repair than the double strand breaks formed by bifunctional alkylating agents.
TopoII inhibitors are often used in combination with other chemotherapy drugs.

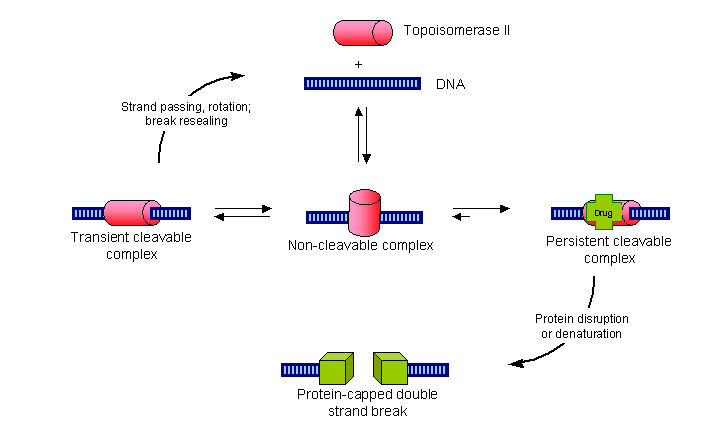
Two classes of natural products have been found which inhibit the process of cell division or mitosis. They are:
| The vinca alkaloids e.g. vincristine |
 |
| The taxoids e.g. taxol |
 |
The M-phase of the cell cycle is subdivided into four sub-phases:
 |
Prophase - nuclear membrane breaks down, mitotic spindle forms, chromosones condense |
 |
Metaphase - chromosones condense at equator |
 |
Anaphase - chromosones segment into chromatids and separate |
 |
Telophase - cell divides into parent and daughter cells |
A crucial component of the cell's machinery for organising and dividing the chromosones are the microtubules. Microtubules are formed by the organised aggregation of dimers of alpha and beta tubulin. Microtubules have a growing end and an end that is constantly breaking down. The balance between growing and shrinking is tightly controlled in cells which allowing the cell to move macromolecules by pushing them with growing microtubules.
Confocal microscope images of cells progressing through the prophase, metaphase, and anaphase show the distribution of the microtubules (green) throughout the M-phase, and how they organise the cell's chromosonal material (blue).
The taxoids also bind to the tubulin dimers at a different site. In this case the formation of abnormal microtubules is promoted. Cell death occurs during the G1 phase and during the G2-M transition and is caused by bundling of accumulated, disorganised microtubule filaments.