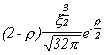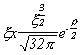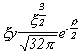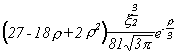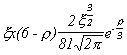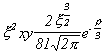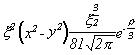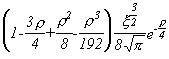|
Selected Atomic Wavefunctions Three columns are presented. The centre column is the wavefunction, including values for all the constants. Students are not expected to learn these: they are presented only to demonstrate to the curious student that the outline forms of the functions given in the right-hand column, in which all constants are represented by A, B, C etc., irrespectively of what they are, are correct. Note that A, B or C represents different things in different lines. Students are expected to learn these outline forms, which will be discussed in lectures. In the centre column, to make the functions appear simpler, ρ (rho) and ξ (xi) are variables defined as ρ = Zr/a0 and ξ = Z/a0 where a0 is the Bohr radius, a physical constant (on the Department of Chemistry Data Card); r is a variable, the radial distance from the nucleus to the point at which the function is to be evaluated (r2 = x2 + y2 + z2, by Pythagoras' Theorem); and Z is the nuclear charge of the atom in units of e.
|