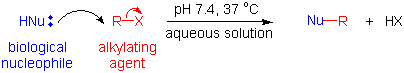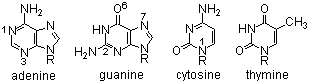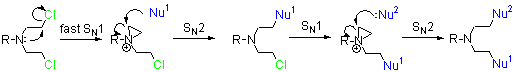Alkylating Agents
General Mechanism of Action
Alkylating agents - replace H with R under physiological conditions.
They react with many biomolecules: water, amino acids, thiols, phosphate.
Alkylation of the heterocyclic bases in DNA results in their cytotoxic and anticancer activity.
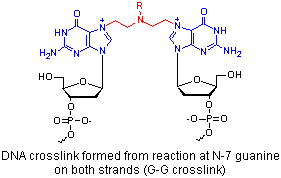
Reaction at guanine (N-7, O-6) > adenine (N-3 > N-1) > cytosine (N-1) >> thymine may result in miscoding during DNA replication (e.g. G-C to G-T shift).
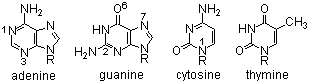
Enzymatic and chemical depurination results in DNA strand breaks.
DNA repair is then initiated, however, if the damage is great enough the damaged cell may trigger suicide or apoptosis.
Nitrogen Mustards
Nitrogen mustard drugs were developed from mustard gas, a war agent first used during the First World War at Ypres.
One of the toxic-effects of exposure is the destruction of the bone marrow and white blood cells. This acute cytotoxicity lead to a period of intense research to find safer, pharmacologically acceptable, analogues which could be used to treat cancer.
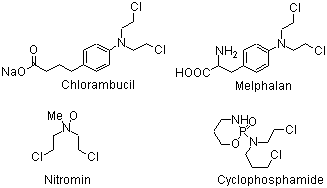
The structures of commonly used mustard drugs are shown below.
Consider the general chemical mechanism of action of nitrogen mustards, how might the rate of the initial SN1 reaction be varied?
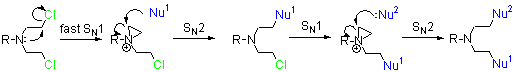
The major cytotoxic event for cells treated with mustard drugs is an interstrand cross-link, usually between two guanines. This produces double strand breaks in DNA.