 Bistable N2–H complexes: The first proposed structure of a H-related colour-causing defect in diamond
Bistable N2–H complexes: The first proposed structure of a H-related colour-causing defect in diamond
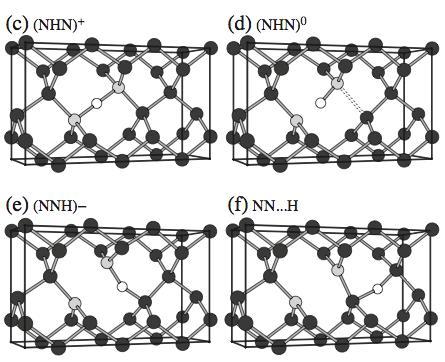
We examine the hydrogenated substitutional nitrogen pair defect, N2–H, in diamond, with different charge states using density functional theory calculations. Neutral N2–H is predicted to exhibit optical absorption in the 600 nm to near infrared range, hence contributing to colour. We suggest that it may be representative of a family of nitrogen-hydrogen complexes, which due to covalent nitrogenhydrogen bonding could explain several observed absorptions. Changing the charge state leads to spontaneous geometric rearrangements, and we find the neutral charge state is thermodynamically metastable. The resultant negative-U behaviour suggests it is unstable as a donor. The structural changes with charge state may contribute to the thermochromic and photochromic behaviour of the so-called 'chameleon' diamonds.
Go back