Home
page
Dr R J Errington
Materials
Chemistry
ROPA
A Molecular Approach to the
Preparation of Thin Borate Films for Integrated Optical
Applications.
Final Report: Grant GR/L72862
Based on preliminary results from this laboratory, this project
was undertaken to develop methods for the synthesis of metal alkoxide
compounds containing boron which might serve as molecular precursors
in the chemical solution deposition (CSD) of thin metal borate films.
As such it provided an opportunity to apply expertise in alkoxide
synthesis and characterisation (both in the solid state and in
solution) to a novel area of materials chemistry in an effort to
understand the factors affecting the properties of the materials
obtained. In the original grant application, funding was requested
for 24 months and the overall aim and individual goals were as
detailed below.
Original Aim of the Project
- To prepare thin films of a range of non-linear optical metal
borate materials from solutions of single-source precursors.
Original Goals:
- To prepare and structurally characterise soluble mixed
metal-boron alkoxides/aryloxides.
- To build a controlled-atmosphere deposition chamber for use
with air-sensitive precursors
- To deposit metal borate films from solutions of molecular
precursors by spin/dip coating.
- To assess the optical properties of borate films produced by
this method.
- To maximise NLO response and lower processing temperatures by
incorporating trigonal borate sites into precursors.
The award of funds for only 18 months required a slight revision
of the aim and goals to those shown below, as much of the materials
work was scheduled for the final 6 months.
Revised Aim of the Project
- To prepare molecular single-source precursors for the
preparation of a range of non-linear optical metal borate
materials by chemical solution deposition.
Goals:
- To build a controlled-atmosphere deposition chamber for use
with air-sensitive precursors.
- To prepare and structurally characterise soluble mixed
metal-boron alkoxides/aryloxides.
- To deposit metal borate films from solutions of molecular
precursors by spin coating.
- To assess the optical properties of borate films produced by
this method.
- To use the "clean box" deposition facilities to produce thin
films of other electroceramic materials, including bismuth
tungstate, by chemical solution deposition.
Achievements:
- A purpose-built facility for chemical solution deposition of
thin electroceramic films has been set up within the PI's
laboratory, enabling materials precursors synthesised in the
laboratory to be assessed without the need for external clean room
or deposition facilities. This capability is already stimulating
and reinforcing interdisciplinary collaborations within the
University and with research groups elsewhere
- A range of novel group 2 metalloborates has been synthesised,
many of which have been characterised by X-ray crystallography.
This has revealed interesting trends in the nature of these
compounds, which can be manipulated by choice of ancillary ligand
on the group 2 metal. 11B NMR parameters have been
correlated with these various types of solid state structures,
providing information about the species present in solutions of
non-crystalline precursors.
- We have established that toluene solutions of a mixed Ba/B
methoxyethoxide precursor, shown by 11B NMR
spectroscopy to contain tetrahedral B and probably a molecular
mixed alkoxide, decompose to b-barium borate much more cleanly
than barium phenoxoborate precursors. The XRD pattern of films
from the methoxyethoxide show preferential 001 oriented
b-barium borate, whereas those from
phenoxide compounds consistently contain extra peaks due to
unidentified material. These results provide the basis for further
investigations and for the optimisation of precursors and
processing conditions.
- The first examples of oriented bismuth tungstate thin films
have been obtained by chemical solution deposition. In work made
possible by the "clean box" deposition facility, films deposited
from a mixed Bi/W 2-ethylhexoxide precursor at relatively low
temperatures (~ 500 oC) are preferentially 010
oriented, demonstrating that this piezoelectric material is an
alternative to PZT for microengineering applications.
In the following sections 1-4, these aspects of the project are
described in greater detail.
1. Installation of a "clean
box" for the preparation of thin films by CSD
Prior to the award of this grant, we relied upon access to
clean-room facilities in other departments in order to prepare our
thin films. This highly unsatisfactory arrangement was hindering
progress in our materials chemistry research and an in-house facility
was urgently required. This grant has enabled us to install a
filtered atmosphere "clean box" in our laboratory for film
deposition, and the schematic diagram in Fig.1 shows its
essential features.
Fig. 1 "Clean Box" for
the preparation of thin films by CSD.
The 3 glove-port box was supplied by Saffron and the furnace was
constructed and fitted to the box in house. The atmosphere inside the
box is not routinely dried, but it is filtered through a HEPA filter
and solvent vapours are removed on an activated charcoal column.
Nitrogen from a boil-off stream is used as the top-up gas. Inside the
box, air-sensitive compounds are handled using Schlenk techniques
with a small vacuum/nitrogen manifold so that precursor solutions can
be used and stored without degradation. Substrates are held on the
spinner with a vacuum chuck and solutions are deposited from a
syringe or dropping pipette before spinning. A hotplate next to the
spinner is used for intermediate drying/calcining between layer
depositions and samples are then transferred through a small door in
the wall of the box to the furnace for higher temperature processing
or final firing, which is carried out under a gas purge (e.g. dry/wet
nitrogen or oxygen). The hotplate and furnace are controlled by
external electronic controllers.
With this facility, films can be deposited as soon as required
after the solution has been prepared in the adjoining laboratory and
we now have much greater control over the whole process. Since its
installation, we have investigated the deposition of barium borate
(BBO), lead germanate (PGO), lead zirconate titanate (PZT) and
bismuth tungstate (BTO) from molecular precursors by CSD. Section 3
summarises the BBO deposition results and the preparation of oriented
BTO films is described for the first time in section
4.
2. Synthetic studies on group 2
metalloborates.
Compounds containing phenoxoborate
[BO(Ph)4]-.
Our initial work in this area showed that the hexanuclear barium
phenoxide
[Ba6(OPh)12(tmeda)2] reacts
with B(OPh)3 in thf to give the mixed phenoxide
[(thf)4BaB2(OPh)8] 1.
This demonstrated that boron aryloxides are sufficiently Lewis acidic
to react with barium aryloxides and give aryloxo-bridge species.
Preliminary film deposition studies using this precursor established
that oriented b-BaB2O4
was formed, but other material was also present in the film.
The main aim of this project was to find alternative precurors that
would decompose cleanly to b-BaB2O4,
so we first had to establish what types of (a) ancillary ligands and
(b) alkoxo ligands could be incorporated into barium aryloxo- and
alkoxoborates. It was soon found that 1 can be obtained readily in
high yield from the more convenient reaction between Ba metal and
phenol in the presence of B(OPh)3. This approach was used
to study the effect of varying the ancillary ether ligands
coordinated to barium.1
Table 1. Barium compounds and their dB(w1/2)
values.a
(click on compound number for picture of structure)
|
|
Compound
|
dB(w1/2)
|
|
1
|
[(thf)4BaB2(OPh)8]
|
1.69 (34)
|
|
2
|
[(dme)2BaB2(OPh)8]
|
2.00 (34)
|
|
3
|
[(diglyme)3Ba][B(OPh)4]2
|
1.85 (9)
|
|
4
|
[(triglyme)2Ba(thf)][B(OPh)4]2
|
1.88 (12)
|
|
5
|
[(tetraglyme)2Ba][B(OPh)4]2
|
1.71 (11)
|
|
6
|
[(12-crown-4)BaB2(OPh)8]
|
1.69 (12)
|
|
7
|
[(18-crown-6)Ba(OPh)2].PhOH
|
&endash;
|
a dB
values in ppm relative to BF3.Et2O; line widths
in Hz.
A series of compounds with polydentate ethers
MeO(CH2CH2O)nMe and crown ethers was prepared
and crystal structures were determined whenever possible (Table 1).
As might have been expected, the coordination geometry of Ba is
sensitive to the nature of the ancillary ligands but, in addition,
these ligands clearly affect the amount of interaction between Lewis
acidic B(OPh)3 and the phenoxo ligands on barium. Fig.
2 illustrates the three possible scenarios in mixtures of
B(OPh)3 and LnB(OPh)2 from
independent species through mixed phenoxide to a barium
tetraphenoxoborate salt. The nature of the structurally characterised
compounds changes from molecular species containing chelating
[B(OPh)4]- ligands
[LnBa{B(OPh)4}2], (1
and 2) with mono- or bidentate ethers to ionic species with
isolated [LnBa]2+ and
[B(OPh)4]- (3-5) with tri- or
tetradentade ethers. In the presence of 18-crown-6 only the barium
phenoxide derivative [(18-crown-6)Ba(OPh)2].PhOH
(7) was isolated.
Fig. 2. Interactions between
B(OPh)3 and LnBa(OPh)2
Solutions of these compounds were studied using 11B NMR
spectroscopy to establish whether their structures were retained in
solution. 11B chemical shifts and line widths are
characteristic of coordination geometry at boron; higher field
signals with narrow line widths being indicative of tetrahedral
boron, whereas trigonal boron gives much broader peaks at lower
field. Compounds 1-6 all gave peaks at ~2 ppm, with larger
line widths for 1 and 2 (~30 Hz) than for 3-6
(~10 Hz), suggesting that the solid state structures are retained in
solution for 1-5 (6 has yet to be fully characterised).
These structures enabled us to correlate the observed 11B
NMR parameters with particular compound types and then use this
correlation to study systems where crystals could not be obtained for
X-ray structural characterisation.
The incorporation of trigonal B centres into
molecular precursors
|
As BBO contains trigonal boron in cyclic
B3O63- rings, we reasoned
that the incorporation of trigonal boron into BBO precursors
may lower processing temperatures. We therefore attempted
the synthesis of compounds containing borate ligands
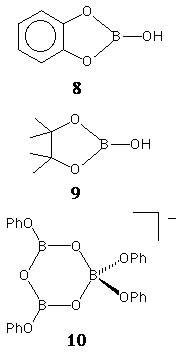
8-10 [e.g. reactions
(1) and
(2) in thf]. Although
results were inconclusive and further work is required in
this area, the 11B NMR spectrum of the product
from reaction (2) contained two
peaks in the region 10-20 ppm, consistent with trigonal
boron.
Ba +2PhOH +
2B3O3(OPh)3 Æ
(1)
Ba{N(SiMe3)2}2 +
(o-C6H4O2)BOH
Æ
(2)
|
|
The synthesis of alkoxide
analogues
Ba + 2MeOH + 2B(OMe)3 Æ
(3)
Ba + 2HOC2H4OMe +
2B(OC2H4OMe)3 Æ
(4)
Ba + HOC2H4OMe + 2B(OEt)3 Æ
(5)
Ba + (HOC2H4)2NH +
2B(OEt)3 Æ
(6)
XRD analysis of films prepared from phenoxide compounds (see
section 3)
showed that decomposition to BBO did not proceed cleanly. Alkoxides
usually have available lower energy decomposition pathways than
aryloxides, so we expected barium alkoxoborates to decompose more
readily than the phenoxoborates. However, only B(OMe)3 had
previously been shown to interact with other metal alkoxides, and all
attempts to obtain methoxo derivatives by reaction
(3) in the presence of various ether
ligands resulted in the formation of insoluble products. Reactions
(4)-(6) were more successful in that
they gave soluble products and, although time constraints did not
allow these compounds to be fully characterised, the 11B
NMR spectrum of the product from reaction (4) contained a peak at -6
ppm with w1/2 20 Hz, indicating that tetrahedral boron was present in
solution. This is the first evidence for the formation of a
tetraalkoxoborate other than [B(OMe)4]-
and suggests that a neutral molecular species
[BaB2{O(CH2)2OMe}8]
11 is present in solution. Note in particular that reaction
(4) was carried out in toluene without
the addition of extra ether ligands, so the methoxy groups of the
alkoxides are presumed to be coordinated to barium in some way. All
attempts to obtain crystals of this compound have been unsuccessful,
but toluene solutions of 11 have been used to deposit thin
films of BBO and XRD analysis results show them to be of higher
quality than those from phenoxoborates (see section
3).
Metalloborates of other group 2
elements
In order to establish whether a similar molecular approach to
other metal borate materials could be adopted, we used a similar
synthetic strategy in our attempts to prepare phenoxoborates of other
group 2 metals. This proved to be successful and we have now prepared
a range of compounds with structurally characterised examples for all
group 2 metals from Mg to Sr in addition to the previously described
barium compounds. Table 2 shows the compounds and their
11B NMR parameters. It is worth noting that in the
11B NMR spectra of magnesium compounds 12 and
13 and the calcium compound 15, the presence of broad,
low field signals indicative of trigonal boron suggests that the
structures are dynamic and undergo dissociation in solution,
particularly in the absence of an excess of ether ligands.
These synthetic, crystallographic and solution NMR studies provide
for the first time a fundamental understanding of the solution
behaviour of group 2 metal alkoxoborates. This knowledge will enable
the properties of molecular precursors to be further optimised for
the solution deposition of b-barium borate
films.
Table 2.
(click on compound number for picture of structure)
|
|
Compound
|
dB(w1/2)
|
|
12
|
[(Et2O)2Mg2B2(OPh)10]
|
1.88 (174), 15.71 (166)
|
|
13
|
[(diglyme)MgB2(OPh)8]
|
1.42 (53), 15.2 (175)
|
|
14
|
[(thf)2CaB2(OPh)8]
|
1.69 (34)
|
|
15
|
[(dme)CaB2(OPh)8]
|
1.49 (21), 15.33 (161)
|
|
16
|
[(thf)6Sr4B2(OPh)14]
|
1.70 (26)
|
|
17
|
[(dme)2SrB2(OPh)8]
|
1.79 (29)
|
|
18
|
[(diglyme)3Sr][B(OPh)4]2
|
1.76 (19)
|
|
19
|
[(triglyme)2Sr][B(OPh)4]2
|
1.70 (16)
|
|
20
|
[(tetraglyme)2Sr][B(OPh)4]2
|
2.00 (20)
|
|
21
|
[(12-crown-4)Sr][B(OPh)4]2
|
1.54 (16)
|
3. Deposition of
b-barium
borate films from barium phenoxo- and alkoxoborates.
Solutions of the metalloborate precursors in toluene or thf were
deposited onto platinised silicon wafers (Pt/Ti/SiO2/Si)
by spin coating at ca. 3000 rpm. In most cases, after
preliminary heating to ~120 oC, the deposition process was
repeated to give three layers before final firing. We were unable to
carry out in situ variable temperature XRD studies on the
diffractometer available, so instead samples were fired at a lower
temperature, taken to the Materials Department for XRD analysis, then
brought back to the laboratory for a higher temperature firing and
the process was repeated for each temperature in the study. For
extended temperature ranges, this laborious process is highly
unsatisfactory, but it did provide sufficient information for us to
assess several samples in the limited time available, and would not
have been possible without the "clean box". Studies of this type
would have been extremely difficult if we were using external clean
room facilities.
Despite these difficulties, we were able to study film deposition
from several of the phenoxide compounds and show that they do not
decompose cleanly to oriented b-barium
borate films. Although samples investigated in this project provided
slightly better quality films than those from our original deposition
studies using solutions of 1, other (as yet unidentified)
peaks were still present in the XRD patterns.
By contrast, methoxyethoxide precursor solutions which we propose
to contain compound 11 gave much better quality films, showing
dominant 104 and 006 reflections in their XRD patterns consistent
with the few previous reports of BBO thin film deposition.2-4
This demonstrates that alkoxo derivatives decompose more readily than
aryloxides and are therefore to be preferred for solution deposition
of oxides. We used no special pre-firing treatment after spinning
these films and samples were fired under a nitrogen atmosphere, but
literature reports3
suggest that improved quality films may be obtained if these samples
were calcined in H2O/O2 at 350 oC
prior to firing.
In an effort to obtain some insight into the solid state
transformations occurring during thermal treatment of the deposited
films, unfired multiple-layer films were submitted to Philips for
variable temperature XRD analysis on state-of-the-art instruments,
but after 8 months the samples have still not been analysed. This
delay has caused much frustration and only serves to emphasise the
need for new in-house powder XRD facilities at Newcastle University
if effective progress is to be made in this and related areas of
materials chemistry.
Due to time constraints, films were only deposited onto platinised
silicon substrates and we were unable to produce sufficient samples
of BBO films for NLO measurements at Durham University. However, now
that we have identified a ligand system which can provide good
quality films we hope to continue these studies and obtain optically
characterised materials on a range of substrates, e.g. glass and
sapphire.
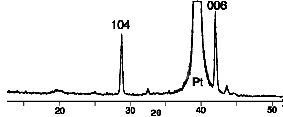
Fig. 3. XRD pattern of BBO from 11 in toluene fired
at 645 oC
4. Deposition of bismuth
tungstate films by CSD
Bismuth tungstate (BTO) is an attractive material for
piezoelectric applications due to its high spontaneous polarisation,
but a reversible phase transition and associated volume change which
occurs at 920-960 oC causes the material to crack on
cooling when prepared by standard high temperature methods. One
advantage of solution deposition is that materials may be obtained at
low temperatures, often in the range 400-600 oC, so we
have been investigating the preparation of bismuth tungstate thin
films by CSD in order to avoid the cracking problems encountered with
other methods. We have previously shown that BTO powders can be
obtained by a sol-gel process from a mixture of tungsten and bismuth
methoxyethoxides5
but only recently has the availability of the "clean box" described
in section 1 now enabled us to prepare the first examples (to our
knowledge) of BTO thin films by chemical solution deposition. Fig. 4
shows the XRD pattern of material deposited on platinised silicon
from Bi(OR)3/WO(OR)4 mixture (ratio = 2:1, OR =
2-ethylhexoxide) in toluene. The 010 oriented films were obtained at
relatively low temperatures (~500 oC), comparable to the
deposition of much studied PZT films. Although further work is
necessary to optimise the processing conditions, these results
demonstrate that Bi2WO6 may be a viable
alternative to PZT for micromechanical piezoelectric applications.
Problems with porosity prevented the electrical characterisation of
our initial films, but ten-layer films are currently being prepared
for electrical characterisation by Prof. R. Whatmore at Cranfield
University.
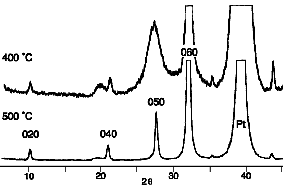
Fig. 4. XRD pattern of BTO from 2-ethylhexoxide
precursors.
References
|
1.
|
R. J. Errington, M. Tombul, G. L. P. Walker, W. Clegg, S.
L. Heath, and L. Horsburgh., J. Chem. Soc., Dalton Trans.,
1999, 3533.
|
|
2.
|
S. Hirano, T. Yogo, K. Kikuta and K. Yamagiwa, J. Am.
Ceram. Soc., 1992, 75, 2590.
|
|
3.
|
T. Yogo, K. Niwa, K. Kikuta, M. Ichida, A. Nakamura and
S. Hirano, J. Mater. Chem., 1997, 7, 929.
|
|
4.
|
D. B. Studebaker, G. T. Stauf, T. H. Baum, T. J. Marks,
H. Zhou and G. K. Wong, Appl. Phys. Lett., 1997, 70,
565.
|
|
5.
|
R. J. Errington and J. Havelock, unpublished results.
|
5. Publications arising from
this work.
|
•
|
Tetraphenoxoborate complexes of barium: crystal
structures of the metalloborates
[Ba(thf)4{B(OPh)4}2]
and
[Ba(dme)2{B(OPh)4}2].
R. J. Errington, M. Tombul, G. L. P. Walker, W. Clegg, S. L.
Heath, and L. Horsburgh., J. Chem. Soc., Dalton
Trans., 1999, 3533-3534.
|
|
•
|
Two further papers on the metalloborates are in
preparation for submission to Inorg. Chem. or
Dalton Trans. early in 2000.
|
|
•
|
In addition, papers are planned on the b-barium
borate and bismuth tungstate thin films once further
characterisation data on samples are available (for
submission to J. Mater. Chem.).
|


