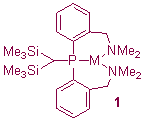
Heteroatom-stabilised carbanions
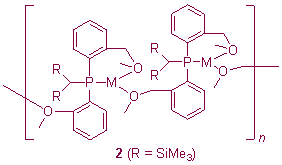
We have a long-standing interest in the chemistry of silicon- and/or phosphorus-stabilised carbanions. Our interest primarily lies in the relationship between structure and reactivity and the effect on these of peripheral donor functionalisation. This functionalisation can have a dramatic effect on ligand deprotonation reactions and binding modes. For example, metalation of the tertiary phosphine {(Me3Si)2CH}P(C6H4-2-CH2NMe2)2 with alkali metal alkyls MeM [M = Li, Na, K, Rb, Cs] yields complexes [{(Me3Si)2C}P(C6H4-2-CH2NMe2)2]M (1),1 in which the amino substituents enforce a PN2-coordination mode, whereby the alkali metal cations bind to the phosphorus rather than the carbanion centres; these represent the first complexes in which a hard heavier alkali metal cation is bound by a soft formal tertiary phosphine. Changing the donor group from NMe2 to OMe results in a significant change in binding mode for the ligand, which favours a bridging mode in its complexes with K and Rb (2).2
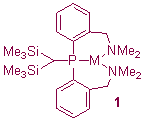

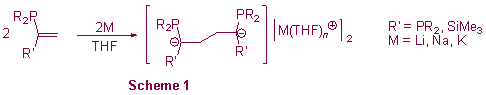
We also have a continuing programme investigating the alkali metal-mediated dimerisation of phosphorus-substituted vinylidenes (Schlenk dimerisation) as a route to novel 1,4-dicarbanions and polyphosphines (scheme 1).3
In a third strand of research we are investigating the structures and reactions of phosphine-borane-stabilised carbanions. These carbanions are key intermediates in the synthesis of numerous important polyphosphines such as the chiral diphosphine DIPAMP (Scheme 2); however, until now there has been no detailed study of these compounds. We have recently initiated a programme of research aimed at elucidating the factors which affect the structures, stabilities and reactions of these compounds. Our initial results suggest that M...H3B contacts are an important feature of these compounds and that the dominance of these contacts can have a substantial effect on their reactivities.4
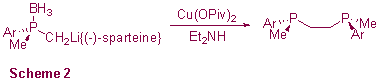
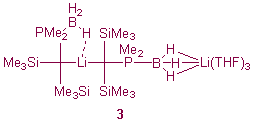
We have recently shown that the complex Li{(Me3Si)2PMe2(BH3)C}2Li(THF)3 (3) crystallises as an unusual contact ion multiple in which there are both Li-C and Li...H3B contacts.
1. (a) W. Clegg, S. Doherty, K. Izod, P. O’Shaughnessy, Chem. Commun. 1998, 1129. (b) W. Clegg, K. Izod, P. O’Shaughnessy, Organometallics 1999, 18, 2939. (c) M. N. S. Hill, K. Izod, P. O’Shaughnessy, W. Clegg, Organometallics 2000, 19, 4531. (d) K. Izod, W. Clegg, S. T. Liddle, Organometallics 2001, 20, 367. (e) K. Izod, P. O’Shaughnessy, W. Clegg, S. T. Liddle, Organometallics 2001, 20, 648. (f) K. Izod, P. O’Shaughnessy, W. Clegg. Organometallics 2002, 21, 641.
2. K. Izod, J. Young, W. Clegg, R. W. Harrington, Dalton Trans. 2005, 1658.
3. (a) W. Clegg, K. Izod, W. McFarlane, P. O’Shaughnessy, Organometallics 1998, 17, 5231. (b) K. Izod, W. McFarlane, B. V. Tyson, W. Clegg, R. W. Harrington, S. T. Liddle, Organometallics 2003, 22, 3684. (c) K. Izod, W. McFarlane, B. V. Tyson, W. Clegg, R. W. Harrington. Dalton Trans. 2004, 4074.
4. K. Izod, C. Wills, W. Clegg, R. W. Harrington, Organometallics, 2006, 25, 38.
Low oxidation state group 14 compounds (tetrylenes)
Since the development of Arduengo-type N-heterocyclic carbenes, the chemistry of divalent heavier group 14 compounds (tetrylenes) has witnessed a dramatic increase in interest. Our interest in this area lies primarily in finding new ways to stabilise heavier tetrylenes through the use of functionalised C- and/or P-donor ligands. Our contributions to this area include the synthesis of a novel dialkylstannylene stabilised by intramolecular B-H...Sn contacts (4) and the development of a range of intramolecularly base-stabilised diphosphatetrylenes (5).5,6
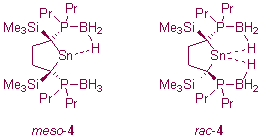
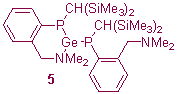
Diasteromeric 4 may readily be separated into its rac and meso forms by a simple recrystallisation procedure. The B-H...Sn contacts in 4 stabilise the electron-deficient Sn(II) centre by between 30 and 40kcal mol-1 and lead to interesting dynamic behaviour in solution.
The diphosphatrylenes 5 are chiral at the two P centres and the tetrel centre and are highly dynamic in solution. Variable temperature NMR experiments demonstrate that the nature of the epimerisation processes for these compounds is dependent on the nature of the tetrel centre and the size of the chelate ring. DFT studies suggest that in certain cases the favoured epimerisation pathway is via an unusual four-coordinate intermediate.
5. K. Izod, W. McFarlane, B. V. Tyson, I. Carr, W. Clegg, R. W. Harrington, Organometallics 2006, 25, 1135.
6. K. Izod, W. McFarlane, B. Allen, W. Clegg, R. W. Harrington, Organometallics 2005, 24, 2157.
Lanthanide organometallic and coordination chemistry
The lanthanide ions are characterised by their large ionic radii, the essentially ionic bonding in their complexes and their tendency to bind hard N- or O-donor ligands. We are interested in the behaviour of complexes of lanthanide(II) and lanthanide(III) ions with soft C- and/or P-donor ligands. We have shown that both homoleptic and heteroleptic alkyl and phosphide complexes are accessible and that these frequently exhibit highly unusual structures and reactivities.
Key results in this area include: (i) the synthesis of the first sigma-bonded organometallic complex of Sm(II) and the isolation of the first crystallographically characterised complex of the benzophenone kettyl radical anion, (ii) the synthesis of some of the first lanthanide(II) phosphide complexes, (iii) isolation of a novel, cyclometalated lanthanum phosphidoalkyl complex (6) and its conversion into a rare cationic organolanthanum(III) compound (7).
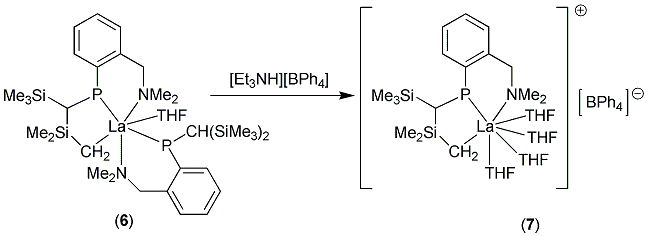
The unique properties of the lanthanide ions frequently lead to rather unusual reactions for our compounds. For example, the reaction between YbI2 and two equivalents of the potassium phosphide [{(Me3Si)2CH}(C6H4-2-OMe)P]K does not give the corresponding Yb(II) bis(phosphide). Instead, the ligand undergoes an unusual rearrangement, generating a heterocubane ytterbium(II) cluster containing an alkoxo-phosphide dianion [[{(Me3Si)2CH}(C6H4-2-O)P]Yb(THF)]4 (8):
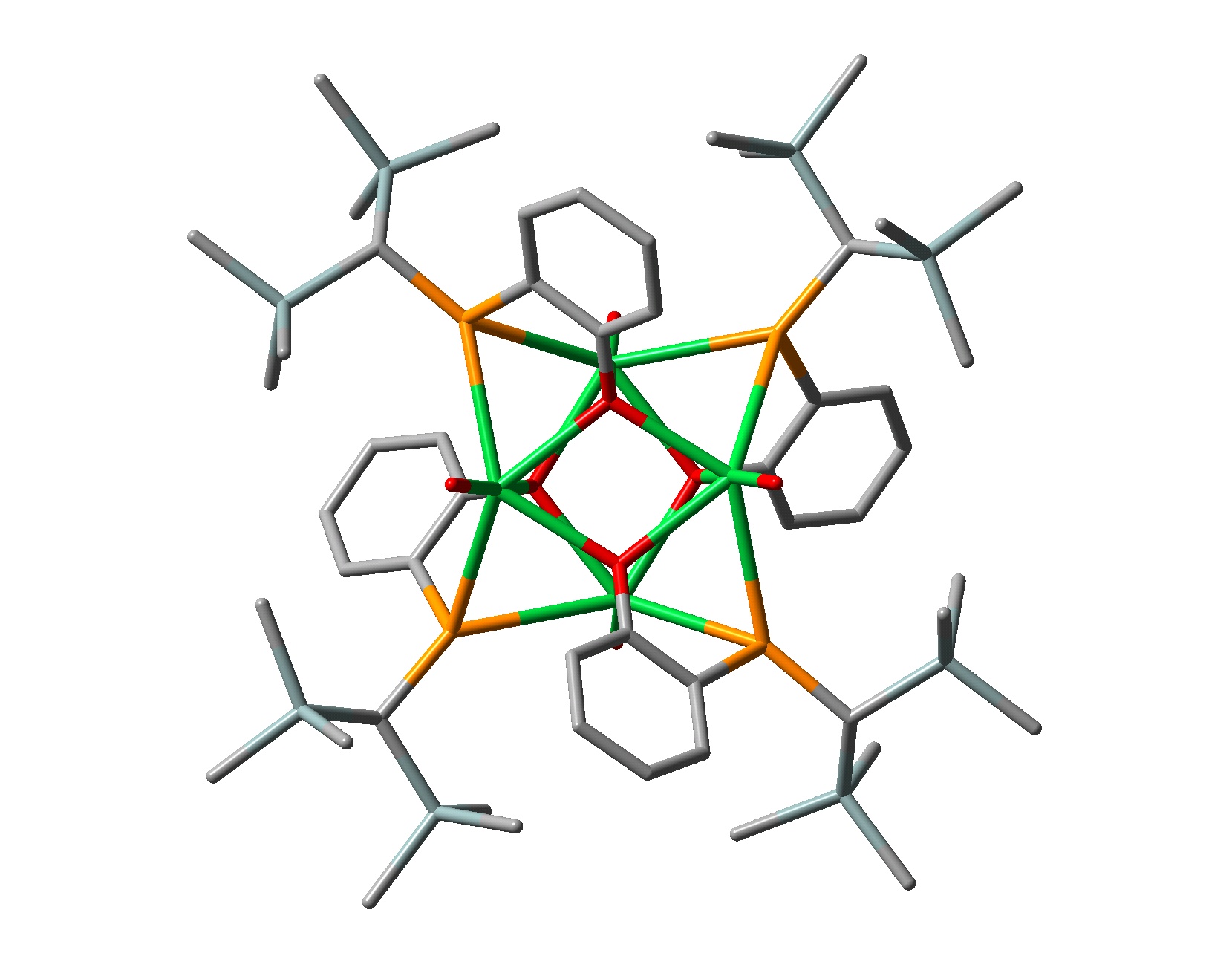
(8)
This is just one example of a wide array of interesting reactions and rearrangements that we have observed in lanthanide alkyl and phosphide complexes.