Professor Bill Clegg:
current research interests
w.clegg@ncl.ac.uk
 X-ray
crystallographic facilities and expertise in Newcastle not only support much of
the synthetic chemistry research within the School and a few external
collaborations, but also help to keep the UK at the forefront of structural
chemistry worldwide through the development and use of internationally leading
synchrotron radiation diffraction techniques. We have run the synchrotron
component of the EPSRC-fund
X-ray
crystallographic facilities and expertise in Newcastle not only support much of
the synthetic chemistry research within the School and a few external
collaborations, but also help to keep the UK at the forefront of structural
chemistry worldwide through the development and use of internationally leading
synchrotron radiation diffraction techniques. We have run the synchrotron
component of the EPSRC-fund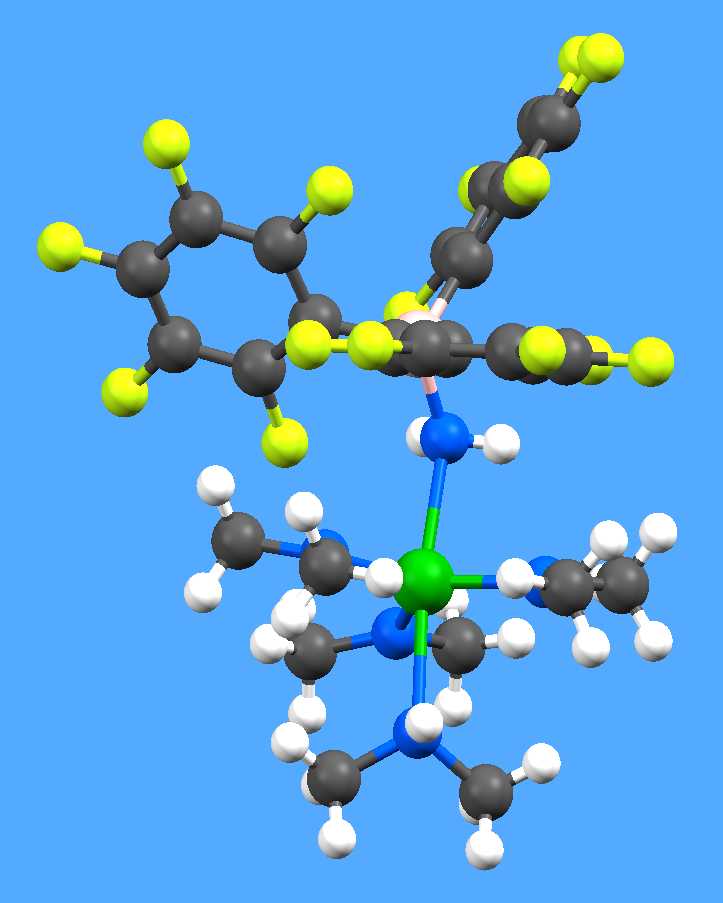 ed National Crystallography Service since 2001, which
currently uses station 9.8 at the Daresbury Laboratory Synchrotron Radiation
Source
with beam-time allocated and funded by STFC. Station 9.8 has recently
celebrated the tenth anniversary of its acclaimed operation as a public user
facility, having been constructed and commissioned in 1994–1997 in a
Newcastle-led project. In 2008, with the closure of SRS, the service will
transfer to the new single-crystal diffraction beamline I19 at Diamond Light
Source, where we are involved in the planning and commissioning.
ed National Crystallography Service since 2001, which
currently uses station 9.8 at the Daresbury Laboratory Synchrotron Radiation
Source
with beam-time allocated and funded by STFC. Station 9.8 has recently
celebrated the tenth anniversary of its acclaimed operation as a public user
facility, having been constructed and commissioned in 1994–1997 in a
Newcastle-led project. In 2008, with the closure of SRS, the service will
transfer to the new single-crystal diffraction beamline I19 at Diamond Light
Source, where we are involved in the planning and commissioning.
Research groups in many UK
University chemistry departments make use of the synchrotron service, which
provides both measured data sets for processing by the users, and fully solved
and refined structures where requested. Popular research areas that make
extensive use of the facilities to study samples that can not generate
acceptable data anywhere else in the UK include supramolecular chemistry,
microporous materials, pharmaceutical research, compounds with special magnetic
and electronic properties, unusual coordination geometries, and catalysis. An
example of the many spectacularly successful results from unpromising samples is
this unprecedented zirconium amidoborate complex from Dr Simon Lancaster at the
University of East Anglia (Chem. Commun. 2005, 2044 and Chem.
Eur. J. 2007, 13, 4535).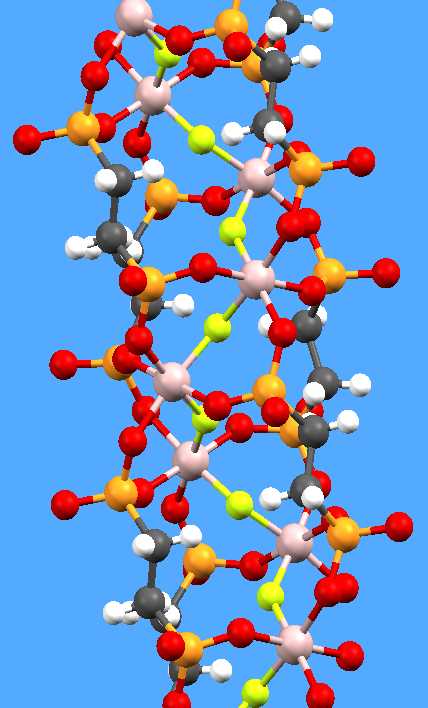
Our own chemical research
includes two principal themes, one of which is supramolecular coordination
chemistry, with polycarboxylates and organophosphonates as ligands. Complexes
of these with various main-group and transition metals form structures ranging
from discrete molecules or anions, through chains an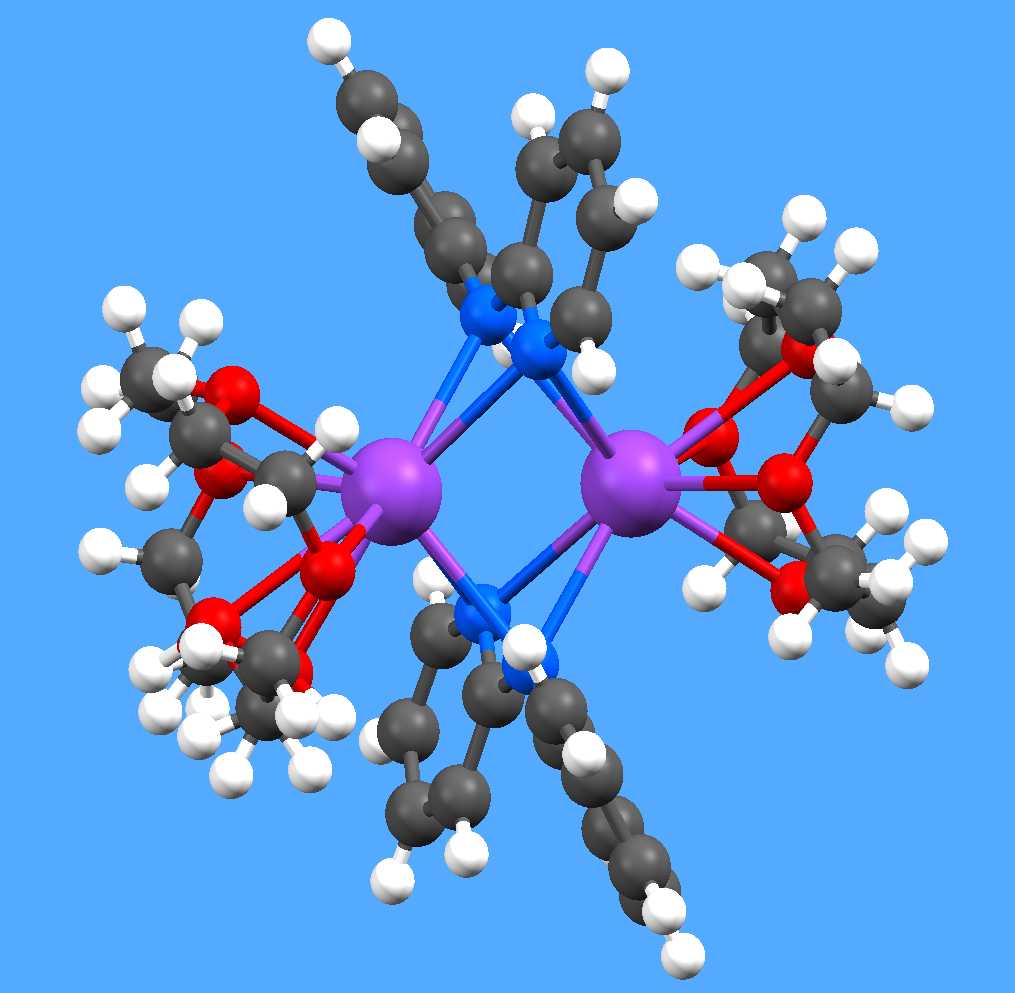 d sheets, to
three-dimensional polymeric networks, many of which involve also hydrogen
bonding and either include solvent molecules or are microporous. The other
principal theme is the structural chemistry of complexes of alkali and alkaline
earth metals with small organic ligands such as pyridones, barbituric acid
derivatives, nucleobases, and other medicinally and biologically relevant
compounds. Many of these also show a complex interplay of metal coordination,
hydrogen bonding and other intermolecular
interactions such as aromatic ring stacking. Examples of both themes are shown
here: a helical aluminium fluoride chain supported by diphosphonate ligands as
pillars (not yet published), and a dimeric potassium-crown complex of a pyridyl
amide (Dalton Trans. 2004, 2514).
d sheets, to
three-dimensional polymeric networks, many of which involve also hydrogen
bonding and either include solvent molecules or are microporous. The other
principal theme is the structural chemistry of complexes of alkali and alkaline
earth metals with small organic ligands such as pyridones, barbituric acid
derivatives, nucleobases, and other medicinally and biologically relevant
compounds. Many of these also show a complex interplay of metal coordination,
hydrogen bonding and other intermolecular
interactions such as aromatic ring stacking. Examples of both themes are shown
here: a helical aluminium fluoride chain supported by diphosphonate ligands as
pillars (not yet published), and a dimeric potassium-crown complex of a pyridyl
amide (Dalton Trans. 2004, 2514).
Examples of results obtained
from samples prepared by other research groups within the Newcastle School of Natural Sciences can be found elsewhere in the School research web pages, mainly
in the Synthesis, Structure and Reactivity section.
Our most productive and
successful external collaboration is a long-standing one with Professor Rab
Mulvey at Strathclyde University, and also involves mainly s-block metal
complexes, some of which additionally incorporate transition metals in a project
investigating alkali-metal-mediated metalation of organic compounds. Several of
the amazing structures discovered in this research have graced the front covers
of journal, including this tetrametalated product of ferrocene (Angew. Chem.
2001, 40, 3902).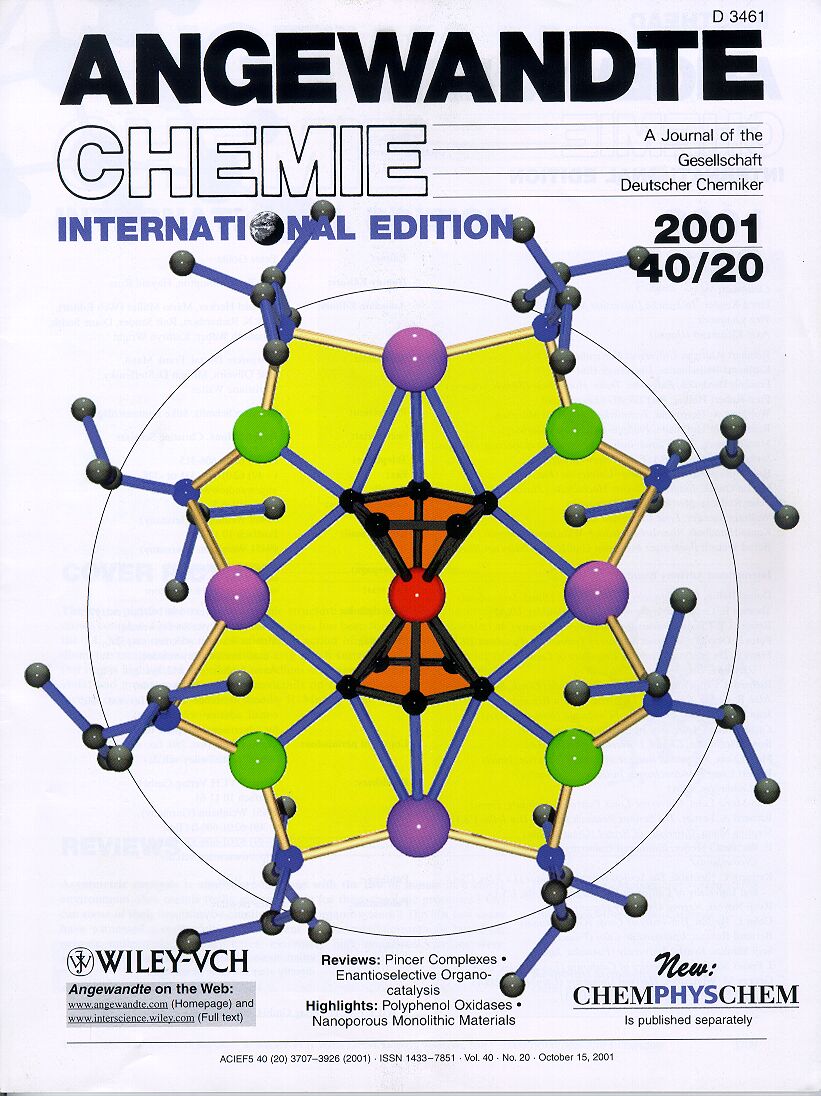
We also undertake a
substantial amount of commercially sponsored structure determination, mostly of
pharmaceutical materials, but also including some compounds with catalytic
applications.
Our own research and numerous collaborations will shortly
be even more strongly supported by the installation of new equipment, funded by
EPSRC, which will include more sensitive detector technology and a combination
of Mo and Cu X-ray sources.
 X-ray
crystallographic facilities and expertise in Newcastle not only support much of
the synthetic chemistry research within the School and a few external
collaborations, but also help to keep the UK at the forefront of structural
chemistry worldwide through the development and use of internationally leading
synchrotron radiation diffraction techniques. We have run the synchrotron
component of the EPSRC-fund
X-ray
crystallographic facilities and expertise in Newcastle not only support much of
the synthetic chemistry research within the School and a few external
collaborations, but also help to keep the UK at the forefront of structural
chemistry worldwide through the development and use of internationally leading
synchrotron radiation diffraction techniques. We have run the synchrotron
component of the EPSRC-fund ed National Crystallography Service since 2001, which
currently uses station 9.8 at the Daresbury Laboratory Synchrotron Radiation
Source
with beam-time allocated and funded by STFC. Station 9.8 has recently
celebrated the tenth anniversary of its acclaimed operation as a public user
facility, having been constructed and commissioned in 1994–1997 in a
Newcastle-led project. In 2008, with the closure of SRS, the service will
transfer to the new single-crystal diffraction beamline I19 at Diamond Light
Source, where we are involved in the planning and commissioning.
ed National Crystallography Service since 2001, which
currently uses station 9.8 at the Daresbury Laboratory Synchrotron Radiation
Source
with beam-time allocated and funded by STFC. Station 9.8 has recently
celebrated the tenth anniversary of its acclaimed operation as a public user
facility, having been constructed and commissioned in 1994–1997 in a
Newcastle-led project. In 2008, with the closure of SRS, the service will
transfer to the new single-crystal diffraction beamline I19 at Diamond Light
Source, where we are involved in the planning and commissioning.
 d sheets, to
three-dimensional polymeric networks, many of which involve also hydrogen
bonding and either include solvent molecules or are microporous. The other
principal theme is the structural chemistry of complexes of alkali and alkaline
earth metals with small organic ligands such as pyridones, barbituric acid
derivatives, nucleobases, and other medicinally and biologically relevant
compounds. Many of these also show a complex interplay of metal coordination,
hydrogen bonding and other intermolecular
interactions such as aromatic ring stacking. Examples of both themes are shown
here: a helical aluminium fluoride chain supported by diphosphonate ligands as
pillars (not yet published), and a dimeric potassium-crown complex of a pyridyl
amide (Dalton Trans. 2004, 2514).
d sheets, to
three-dimensional polymeric networks, many of which involve also hydrogen
bonding and either include solvent molecules or are microporous. The other
principal theme is the structural chemistry of complexes of alkali and alkaline
earth metals with small organic ligands such as pyridones, barbituric acid
derivatives, nucleobases, and other medicinally and biologically relevant
compounds. Many of these also show a complex interplay of metal coordination,
hydrogen bonding and other intermolecular
interactions such as aromatic ring stacking. Examples of both themes are shown
here: a helical aluminium fluoride chain supported by diphosphonate ligands as
pillars (not yet published), and a dimeric potassium-crown complex of a pyridyl
amide (Dalton Trans. 2004, 2514).