Journal Articles
- Rothwell KA, Pentrak MP, Pentrak LA, Stucki JW, Neumann A. Reduction Pathway-Dependent Formation of Reactive Fe(II) Sites in Clay Minerals. Environmental Science & Technology 2023, 57, 10231-10241. doi: 10.1021/acs.est.3c01655
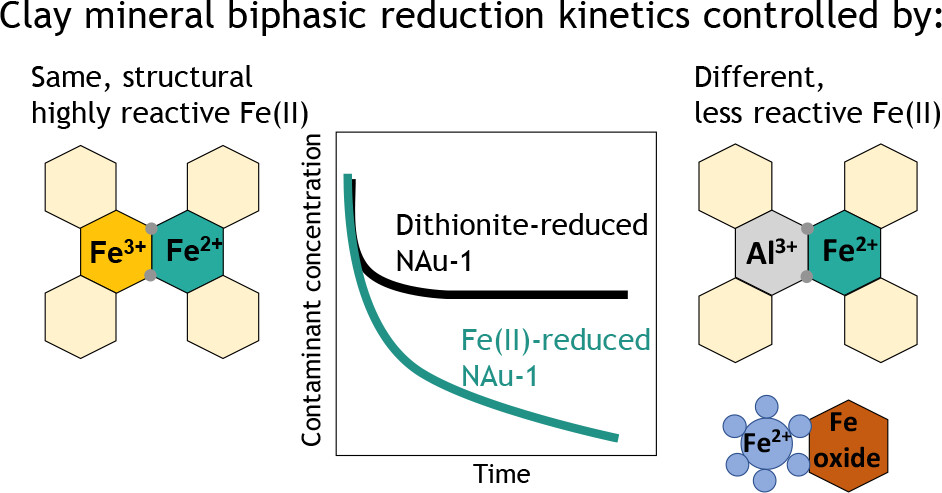
- Vasilopanagos C, Carteret C, Hillier S, Neumann A, Brooksbank HJL, Greenwell HC. Effect of Structural Fe Reduction on Water Sorption by Swelling and Non-Swelling Clay Minerals. Minerals 2022, 12, 4, 453. doi: 10.3390/min12040453
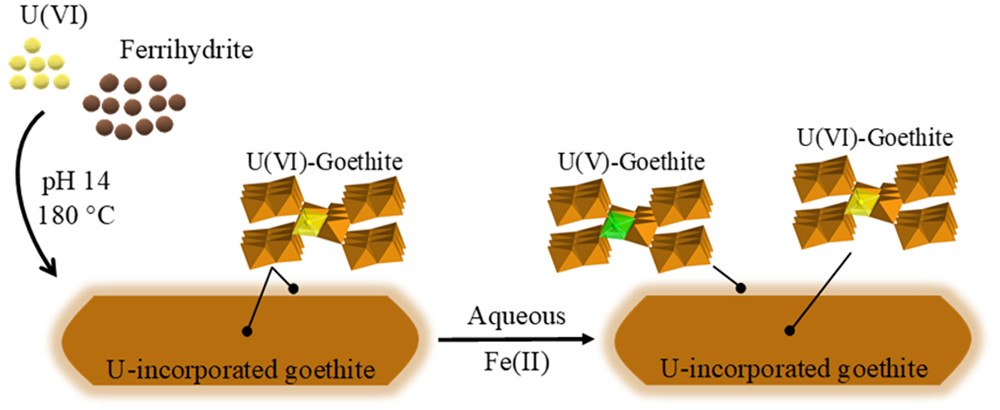
- Stagg O, Morris K, Lam A, Navrotsky A, Velázquez JM, Schacherl B, Vitova T, Rothe J, Galanzew J, Neumann A, Lythgoe P, Abrahamsen-Mills L, Shaw S. Fe(II) Induced Reduction of Incorporated U(VI) to U(V) in Goethite Environmental Science & Technology 2021, 55, 24, 16445–16454. doi: 10.1021/acs.est.1c06197
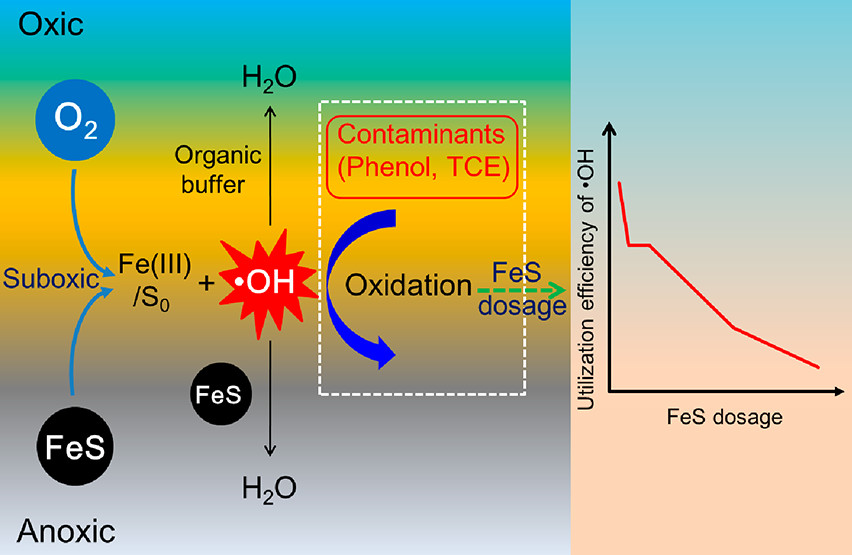
- Cheng D, Neumann A, Yuan SH, Liao WJ, Qian A. Oxidative Degradation of Organic Contaminants by FeS in the Presence of O2. Environmental Science & Technology 2020, 54, 7, 4091-4101. doi: 10.1021/acs.est.9b07012
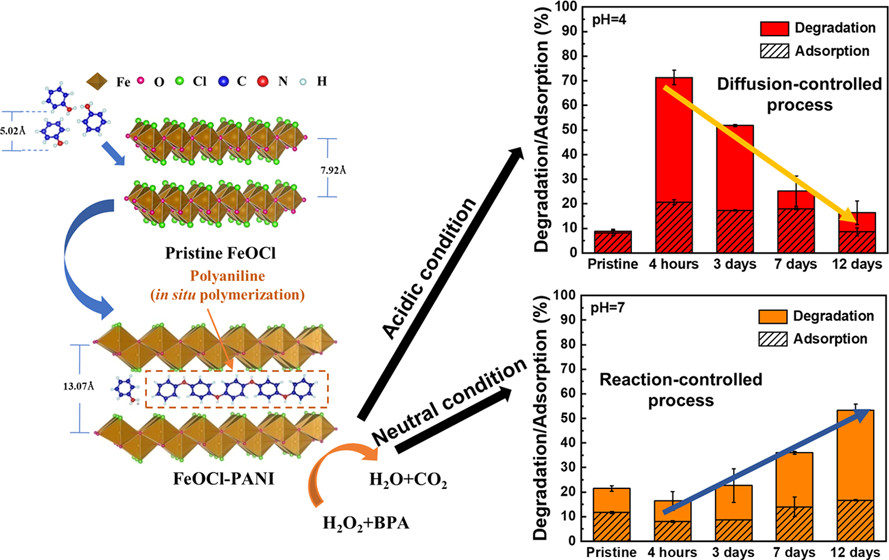
- Wang J, Tsai, M-C, Lu Z, Li Y, Huang G, Wang H, Liu H, Liao X, Hwang B-J, Neumann A, Yang X. pH-dependent structure-activity relationship of Polyaniline-intercalated FeOCl for heterogeneous Fenton reactions. ACS Omega 2019, 4, 26, 21945-21953. doi:10.1021/acsomega.9b03008
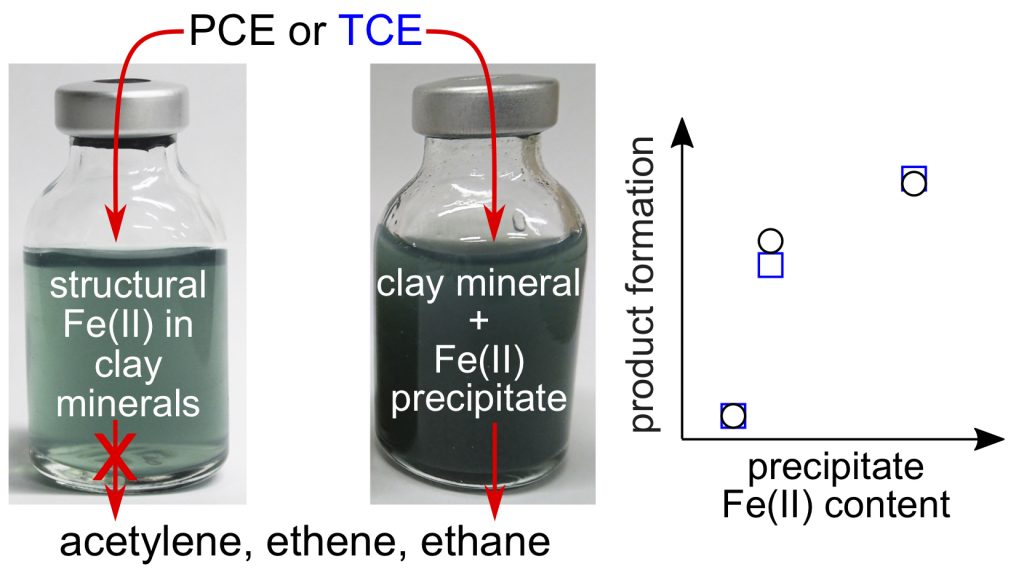
- Entwistle J, Latta DE, Scherer MM, Neumann A. Abiotic Degradation of Chlorinated Solvents by Clay Minerals and Fe(II): Evidence for Reactive Mineral Intermediates. Environmental Science & Technology 2019, 53, 24, 14308-14318. doi: 10.1021/acs.est.9b04665
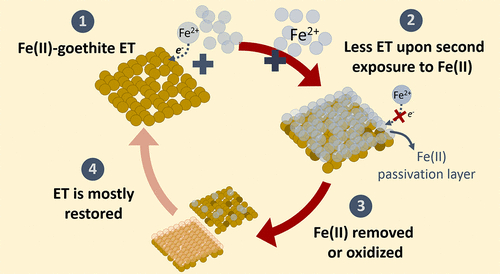
- Notini L, Latta DE, Neumann A, Pearce, CI, Sassi M, N’Diaye AT, Rosso KM, Scherer MM. A Closer Look at Fe(II) Passivation of Goethite. ACS Earth and Space Chemistry 2019, 3, 2717–2725. doi:10.1021/acsearthspacechem.9b00224
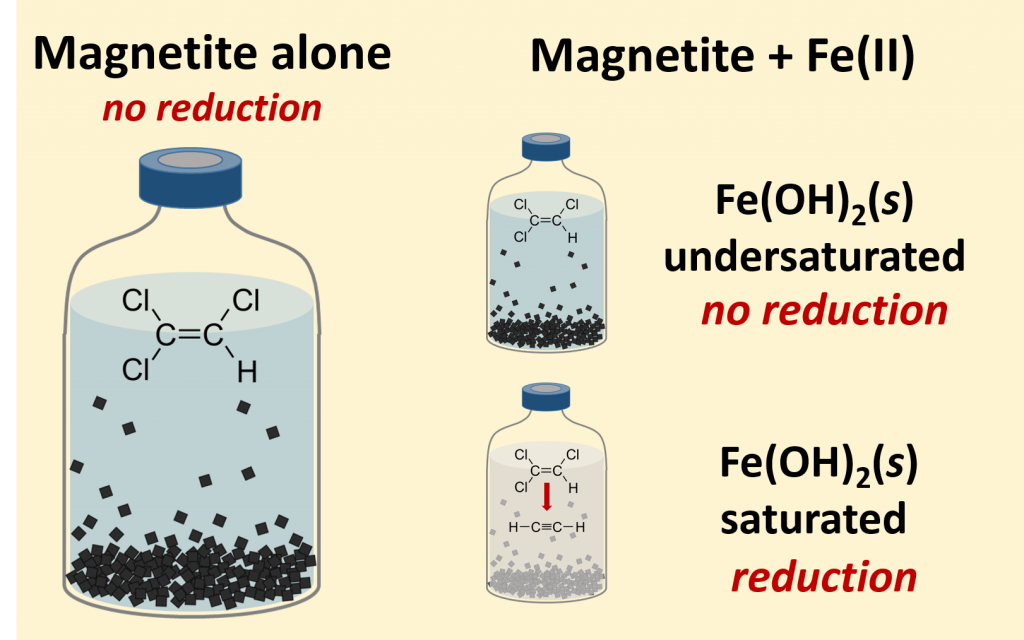
- Culpepper JD, Scherer MM, Robinson TC, Neumann A, Cwiertny D, Latta DE. Reduction of PCE and TCE by Magnetite Revisited. Environmental Science: Processes and Impact 2018, 20, 1340-1349. doi:10.1039/C8EM00286J
- Notini L, Latta DE, Neumann A, Pearce CI, Sassi M, N’Diaye AT, Rosso KM, Scherer MM. The Role of Defects in Fe(II)-Goethite Electron Transfer. Environmental Science & Technology 2018, 52(5), 2751–2759. doi:10.1021/acs.est.7b05772
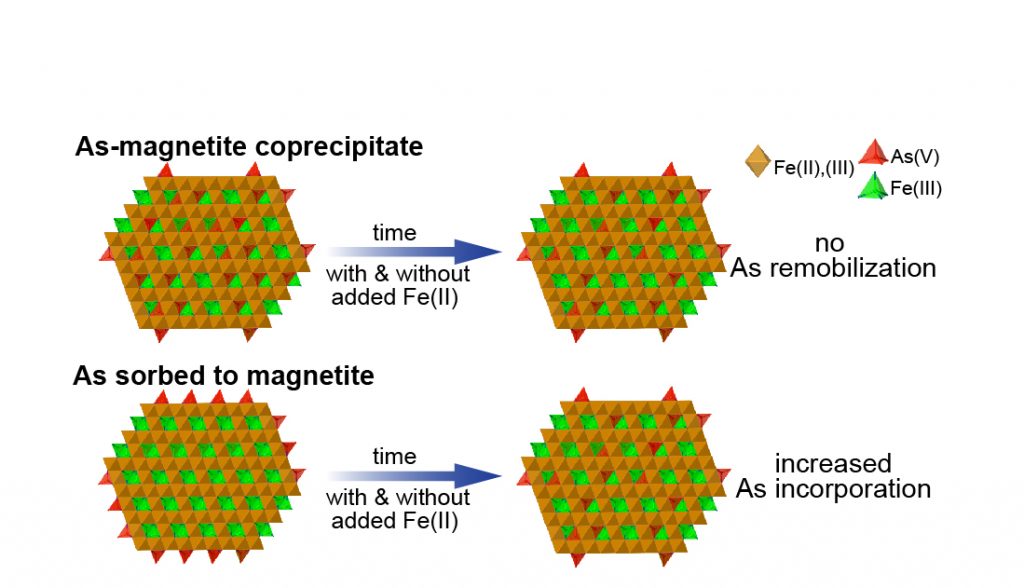
- Huhmann BL, Neumann A, Boyanov MI, Kemner KM, Scherer MM. As(V) in Magnetite: Incorporation and Redistribution. Environmental Science: Processes and Impact 2017, 19, 1208-1219. doi:10.1039/C7EM00237H
- Qafoku O, Pearce C, Neumann A, Kovarik L, Zhu M, Ilton E, Bowden M, Resch C, Arey B, Arenholz E, Felmy A, Rosso K. Tc(VII) and Cr(VI) Interaction with a Naturally Reduced Ferruginous Smectite from the Hanford Redox Transition Zone Environmental Science & Technology 2017, 51 (16), 9042–9052. doi:10.1021/acs.est.7b02191
- Latta DE, Neumann A, Premaratne WAPJ, Scherer MM. Fe(II)-Fe(III) electron transfer in a clay mineral with low Fe content. ACS Earth and Space Chemistry 2017, 1 (4), 197–208. doi:10.1021/acsearthspacechem.7b00013
- Neumann A, Wu L, Li W, Beard BL, Johnson CM, Rosso KM, Frierdich AJ, Scherer MM. Atom exchange between aqueous Fe(II) and structural Fe in clay minerals. Environmental Science & Technology 2015, 49(5), 2786–2795. doi:10.1021/es504984q
- Handler RM, Frierdich AJ, Johnson CM, Rosso KM, Beard BL, Wang C, Latta DE, Neumann A, Pasakarnis T, Premaratne WAPJ, Scherer MM. Fe(II)-Catalyzed Recrystallization of Goethite Revisited. Environmental Science & Technology 2014, 48(19), 11302–1131. doi:10.1021/es503084u
- Neumann A, Kaegi R, Voegelin A, Hussam A, Munir AKM, Hug SJ. Arsenic removal with composite iron matrix filters in Bangladesh: a field and laboratory study. Environmental Science & Technology 2013, 47(9), 4544-4554. doi:10.1021/es305176x
- Alexandrov V, Neumann A, Scherer MM, Rosso KM. Electron Exchange and Conduction in Nontronite from First-Principles. Journal of Physical Chemistry C 2013, 117(5), 2032-2040. doi:10.1021/jp3110776
- Neumann A, Olson TL, Scherer MM. Spectroscopic Evidence for Fe(II)–Fe(III) Electron Transfer at Clay Mineral Edge and Basal Sites. Environmental Science & Technology 2013, 37(13), 6969-6977. doi:10.1021/es304744v
- Neumann A, Petit S, Hofstetter TB. Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy. Geochimica et Cosmochimica Acta 2011, 75(9), 2336-2355. doi:10.1016/j.gca.2011.02.009
- Neumann A, Hofstetter TB, Skarpeli-Liati M, Schwarzenbach RP. Reduction of polychlorinated ethanes and carbon tetrachloride by structural Fe(II) in smectites. Environmental Science & Technology 2009, 43(11), 4082-4089. doi:10.1021/es9001967
- Neumann A, Hofstetter TB, Lüssi M, Cirpka OA, Petit S, Schwarzenbach RP. Assessing the redox reactivity of structural iron in smectites using nitroaromatic compounds as kinetic probes. Environmental Science & Technology 2008, 42(22), 8381-8387. doi:10.1021/es801840x
- Hofstetter TB, Neumann A, Arnold WA, Hartenbach AE, Bolotin J, Cramer CJ, Schwarzenbach RP. Substituent effects on nitrogen isotope fractionation during abiotic reduction of nitroaromatic compounds. Environmental Science & Technology 2008, 42(6), 1997-2003. doi:10.1021/es702471k
- Hofstetter TB, Neumann A, Schwarzenbach RP. Reduction of nitroaromatic compounds by Fe(II) species associated with iron-rich smectites. Environmental Science & Technology 2006, 40(1), 235-242. doi:10.1021/es0515147
Book Chapters
- Neumann A, Sander M, Hofstetter TB. Redox Properties of Structural Fe in Smectite Clay Minerals. In: Tratnyek, PG; Grundl, TJ; Haderlein, SB, ed. Aquatic Redox Chemistry. Washington DC: American Chemical Society, 2011, pp.361-379. doi:10.1021/bk-2011-1071.ch017