Antibody-based PET imaging agents
Why antibodies?
Antibodies are large proteins that protect your body from infection. Normally, an antibody’s main job is to bind to things like viruses and bacteria and remove them from the body.
My research involves antibodies which can bind to proteins which are located on the surface of cancer cells with exquisitely high sensitivity and specificity.
By attaching a positron-emitting radioisotope to these antibodies, this allows us to see exactly where the antibodies are travelling to in the body using PET. This can provide crucial information such as the location of any tumours in the body and what sort of treatments will work best.
One important positron-emitting radioisotope which is perfect for this work is zirconium-89 – read on to find out more about this radioisotope and how it is attached to an antibody.
Radiosynthesis of zirconium-89 labelled antibodies
The radiometal zirconium-89 is ideal for producing antibody-based imaging agents that can be tracked with PET. This isotope undergoes radioactive decay with a half-life of 3.3 days which is very compatible with the length of time that antibodies reside within the body. Its radioactive emissions also result in clear and easily quantifiable images.
Unfortunately, we can’t just attach zirconium-89 (Zr-89) straight on to an antibody as it would quickly detach and travel elsewhere in the body – mostly to bones. And so, we first have to modify the antibody with a type of molecule called a ‘chelating agent’ (pronounced kee-lating agent rather than chee-lating agent). This word is of Greek origin and means “claw” – this is an apt description as chelating agents are designed to grip on tightly to metal isotopes without letting go. In the case of zirconium-89, the most commonly used chelating agent is called deferoxamine (DFO). To attach the DFO to an antibody, we use a bifunctional derivative of DFO which contains an isothiocyanate group which reacts with primary amines located on the antibody (typically found on lysine residues), resulting in the formation of a thiourea linkage. At this stage, we can add the zirconium-89 which will then bind tightly to the DFO-modified antibody. During my previous post-doctoral position at Oxford University (advisor: Prof. Bart Cornelissen) we succeeded in miniaturizing this whole process to allow us to work with microgram amounts of antibodies. This is really important as antibodies can be very expensive – especially good ones which bind to newly discovered targets!!
You can read more about this in our recent papers on this topic, here, here, here, and here!
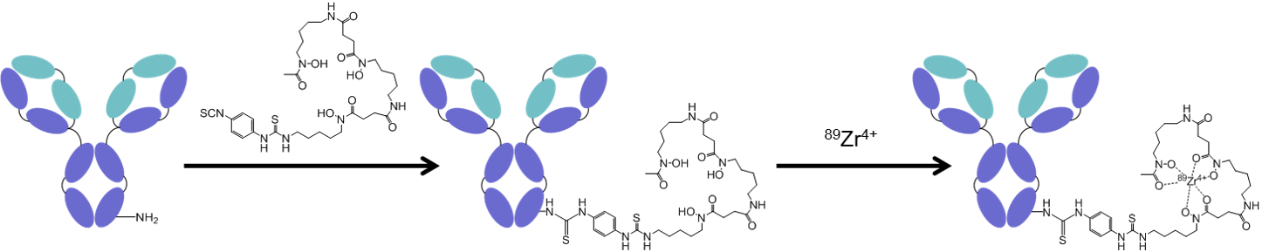 The process by which most 89Zr-labelled antibodies are synthesised
The process by which most 89Zr-labelled antibodies are synthesised